Why Does It Take So Long to Find a Cure for COVID-19?
This article, in a slightly edited form, first appeared on Pain News Network on April 18, 2019.
Americans love to hate Pharma; its greed and self-interest is evident. The nearly doubling insulin price makes it unaffordable for many, and the 5,000 percent price increase of an infection-fighting drug, Daraprim, are just two examples of avarice that have fueled the loathing of the pharmaceutical industry.
However, if we are going to find a cure for COVID-19, it will come from Pharma. These days, we hear fewer complaints about greed and more inquiries about speed. Why can’t we find a cure for COVID-19 more quickly?
Understanding the Drug Development Process
Understanding the process of drug development may not change anyone’s attitude towards Pharma, but it can help us understand why we’ll be waiting awhile for COVID-19 treatments and vaccines.
Drug development begins with trying to find a substance that has the potential to treat or prevent a disease. This is called the discovery process. Once a drug candidate is chosen, it must be studied in animals to determine its potential risk to humans. If the risks appear acceptable, human clinical trials can begin.
When a candidate therapy is ready for a Phase I human trial, it must be approved by the U.S. Food and Drug Administration (FDA). Initial doses given to volunteers are very low. Then they are gradually and methodically increased until side effects develop. This allows researchers to determine a reasonably safe potential therapeutic dose.
Phase I trials for COVID-19 drugs and vaccines are frequently discussed in the news as if these tests can determine their effectiveness. However, Phase I studies only evaluate safety. They don’t reveal how well the drug will work.
If a drug appears to be reasonably safe on the basis of a Phase I study, it moves to Phase II. This is the first time a drug is given to see if it has a signal for efficacy. Unfortunately, most drugs fail to demonstrate safety in Phase I and in Phase II trials. The few drugs that survive Phases I and II advance to Phase III. Most Phase III trials involve testing approximately 1,000 subjects, and usually take 1 to 2 years to complete.
Only 14 percent of drugs that enter Phase I clinical trials are ultimately approved by the FDA.
Fast-Tracking Drugs to Beat the Pandemic
If drugs successfully pass all three test phases, the results of the studies are submitted to the FDA for approval.
On February 29, the FDA established an accelerated process for potential COVID-19 therapies and vaccines. In addition drugs, can be accepted to be studied via the FDA’s Fast Track path. The figure below shows the average length of time it takes to conduct the normal phases of drug development (up to 15 years) compared to the Fast Track process (up to 5.5 years). The new accelerated path for COVID-19 therapies should be much shorter. The figure also shows how many research studies for COVID-19 treatments were in each stage of development as of April 11, 2020.
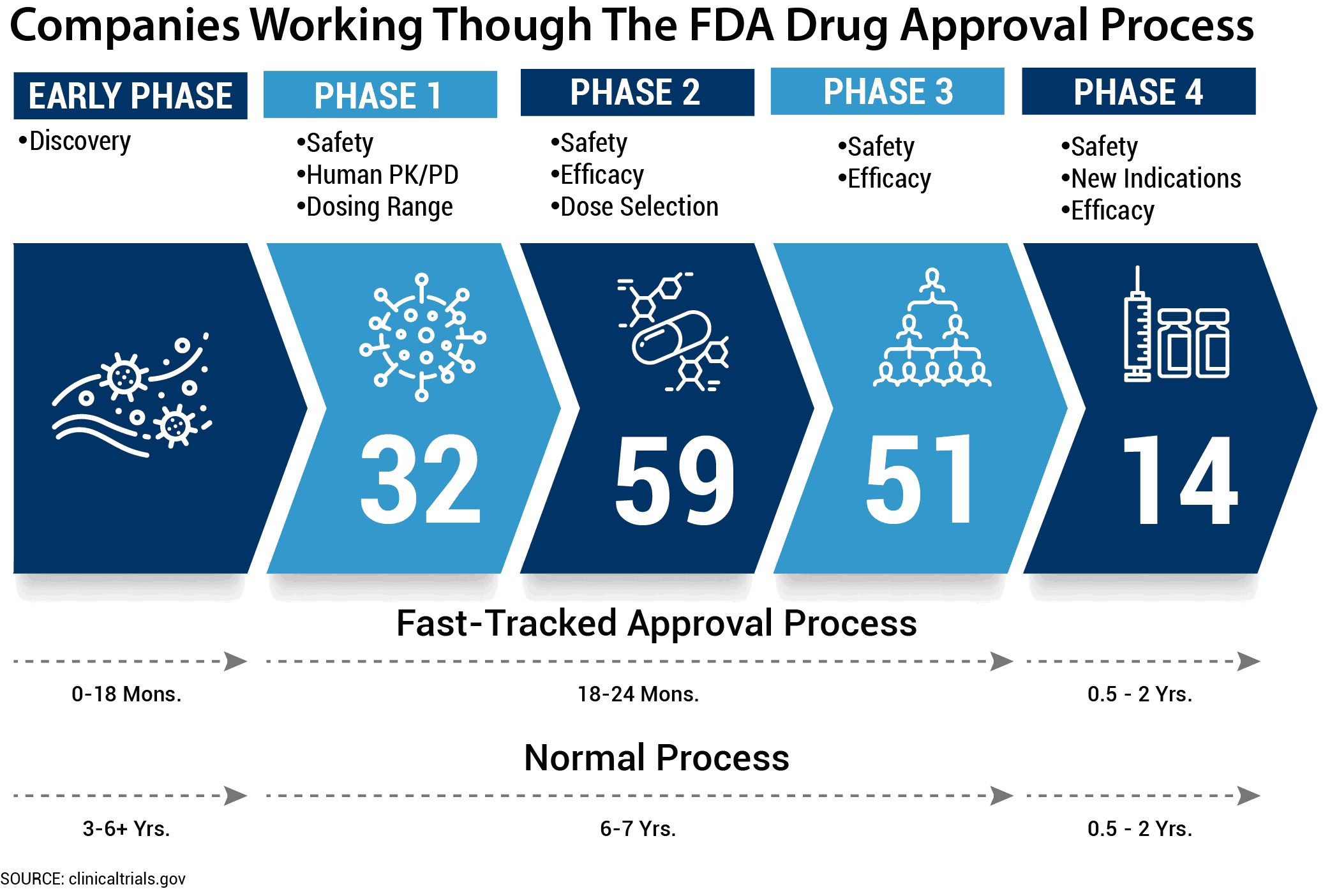
Credit: Stephen Reich
In summary, the National Institutes of Health (through clinicaltrials.gov) reports there are 156 COVID-19 treatments in the pipeline, some of which could take up to 5.5 years to produce viable treatment. That doesn’t sound very helpful for today’s crisis.
However, some already-approved drugs can be studied for new applications. This usually occurs in Phase IV studies. Hydroxychloroquine, remdesivir, and tocilizumab are three examples of drugs approved for other uses that are currently being studied as possible treatments for COVID-19. Results from studies using already approved drugs can be available within a few months.
But we need to know that, when these drugs are repurposed, they are safe for the new use. That is not always the case. For example, Brazil ended a recent chloroquine study because the drug seemed to be causing coronavirus patients to have irregular heartbeats.
Pharma Deserves Our Full Support Now
Currently, there is no cure or vaccine proven to be effective against COVID-19. There simply hasn’t been enough time to conduct the required research. However, as the above figure illustrates, there is a gallant worldwide effort to find effective treatments and vaccines.
Now is the time for Pharma to use its extraordinary expertise to provide the world with effective treatments for this pandemic. Although antipathy toward Pharma may remain, this is the time to cheer for their success. Our lives may depend on it.
Lynn R. Webster, MD, is a vice president of scientific affairs for PRA Health Sciences and consults with the pharmaceutical industry. He is author of the award-winning book, “The Painful Truth,” and co-producer of the documentary, “It Hurts Until You Die.” Opinions expressed here are those of the author alone and do not reflect the views or policy of PRA Health Sciences.
You can find him on Twitter: @LynnRWebsterMD.
Thanks for this explanation Lynn. I continue to learn from you.